+1 604 900 5368
Continuous Quality Improvement (CQI)
Colleges of Pharmacists require mandatory anonymous medication incident reporting in all pharmacies using any medication incident reporting platform of the pharmacy’s choosing that meets each provincial College's respective criteria.
Overarching Goals:
-
To ensure College requirements enhance public care and safety
-
Develop and implement Medication Incident Reporting (MIR) to identify trends in incidents across the province and to identify opportunities to learn and improve practice and health outcomes
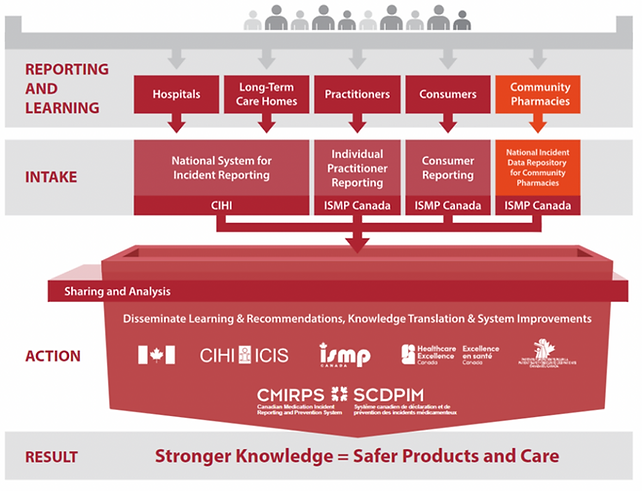
Any MIR platform must also integrate with a national/provincial database to share anonymous and de-identified medication incident and near miss reports.
Highlights of National Standards for Continuous Quality Improvement (CQI):
-
Foster culture of patient safety and a just culture in the workplace to promote learning and quality improvement
-
Identify root causes and contributing factors for medication incidents and near misses
-
Follow-up with team members involved in medication incidents and near misses, encourage peer support when appropriate
-
Review and update pharmacy policies and procedures based on root cause analyses, safety self-assessments, summary reports and data
-
Implement and monitor improvements to the pharmacy’s procedures in accordance with pharmacy’s continuous quality improvement plan
36Eight Technologies and its platform C/R/I/S is Institute of Safe Medication Practices (ISMP) Canada, Continuous Quality Improvement (CQI), compliant

C/R/I/S
-
MIR platform interfacing with national databases
-
Sharing anonymous and de-identified reports
.png)